Research Articles
The Ubiquitin-Proteasome System: From Molecular Mechanism to Therapeutic Application in Disease and Drug Development
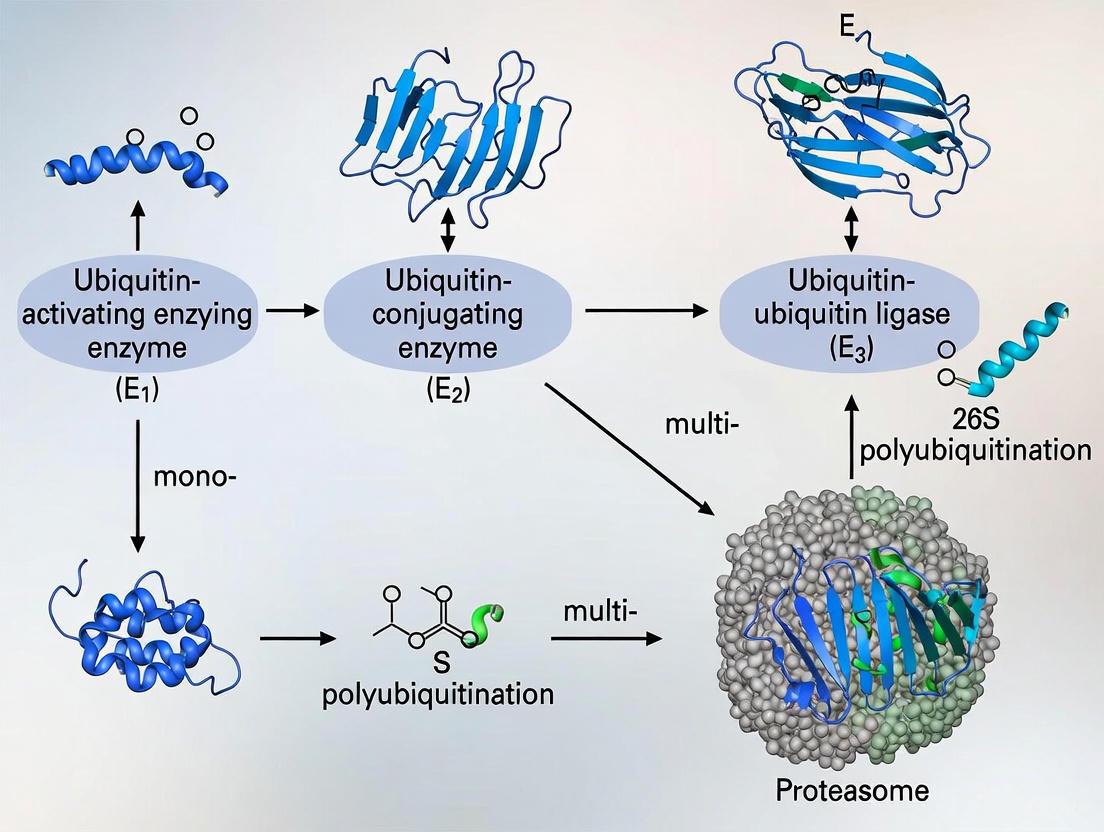
This article provides a comprehensive exploration of the ubiquitin-proteasome system (UPS), a crucial pathway for intracellular protein degradation and regulation.
Advanced Methods for Discovering Novel Ubiquitination Sites: A Guide for Researchers and Drug Developers
This article provides a comprehensive overview of the evolving methodologies for discovering novel protein ubiquitination sites, a critical post-translational modification with vast implications in cell regulation and disease.
Beyond K48 and K63: The Emerging Functions of Atypical Ubiquitin Chains K6, K27, and K29 in Cell Signaling and Disease
This review synthesizes current knowledge on the structures, biological functions, and regulatory mechanisms of the atypical ubiquitin chain linkages K6, K27, and K29.
Proteasome Inhibition and Ubiquitin Homeostasis: Mechanisms, Measurement, and Clinical Implications in Disease Therapy
This article provides a comprehensive analysis of how proteasome inhibition directly impacts cellular ubiquitin levels and dynamics, a critical consideration in both basic research and drug development.
Ubiquitin Phosphorylation: Decoding a Novel Regulatory Layer in Cellular Signaling and Disease
This article synthesizes the latest advances in understanding ubiquitin phosphorylation, a pivotal post-translational modification that expands the ubiquitin code.
Strategies for Low Abundance Ubiquitinated Protein Identification: Enrichment, MS Methods, and Clinical Applications
Identifying low abundance ubiquitinated proteins is a critical challenge in proteomics, essential for understanding cellular regulation, disease mechanisms, and drug discovery.
Decoding the Ubiquitin Chain Architecture: Detection Challenges and Advanced Methodologies
This article provides a comprehensive overview of how the complex architecture of ubiquitin chains—including homotypic, mixed, and branched topologies—presents significant challenges for detection and characterization.
Deubiquitinating Enzymes: Gatekeepers of Ubiquitin Homeostasis in Health and Disease
This article provides a comprehensive analysis of the critical role Deubiquitinating Enzymes (DUBs) play in maintaining ubiquitin homeostasis, a process fundamental to cellular health.
Ubiquitination Stoichiometry: Unraveling the Quantitative Challenge in Cellular Signaling and Drug Development
This article provides a comprehensive analysis of ubiquitination stoichiometry—the fraction of a specific protein molecule that is ubiquitinated at a given site.
Endogenous Ubiquitination Detection: Overcoming Key Challenges in Basic Research and Drug Development
Accurately detecting endogenous protein ubiquitination is a cornerstone for understanding cellular regulation and developing targeted therapies like PROTACs.